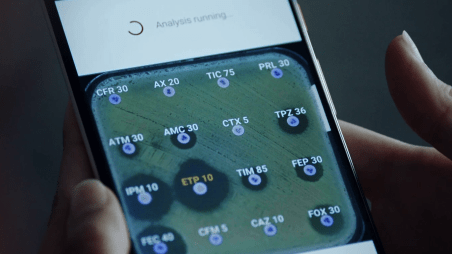
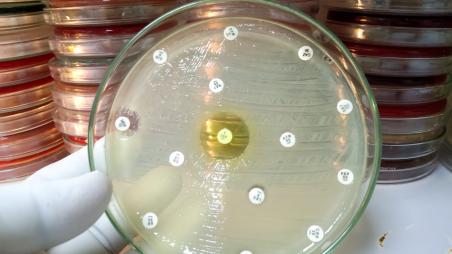
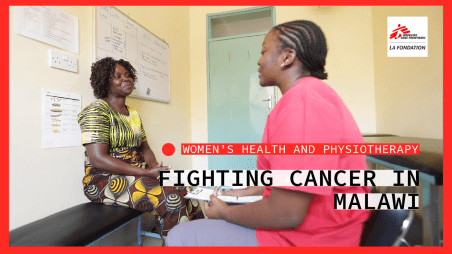
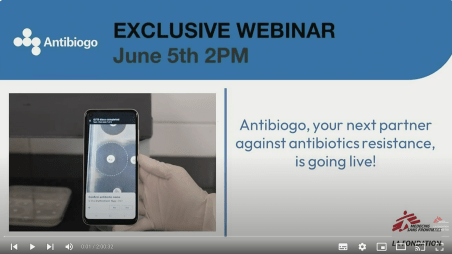
Newsroom
Headline

Incontinence, Pain, Isolation: could physiotherapy change the lives of mothers? Insights from Yemen
Launch of Clinical Studies for a Rapid Measles Diagnostic Test – DiaTROPIX
MSF Foundation in Video
News
Publications
Press Review
No news for now.