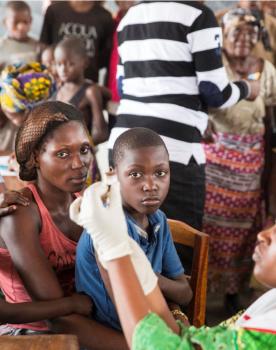
Oliver Barth/MSF
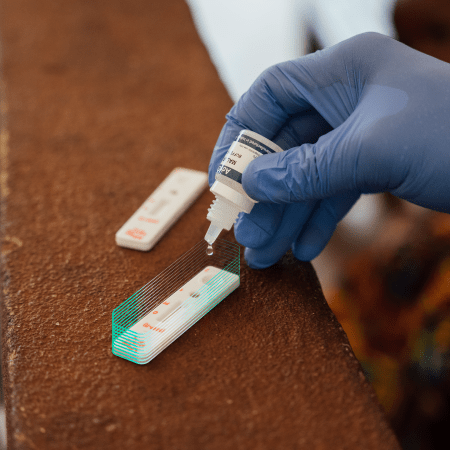
RDTs : Measles and meningitis
In brief
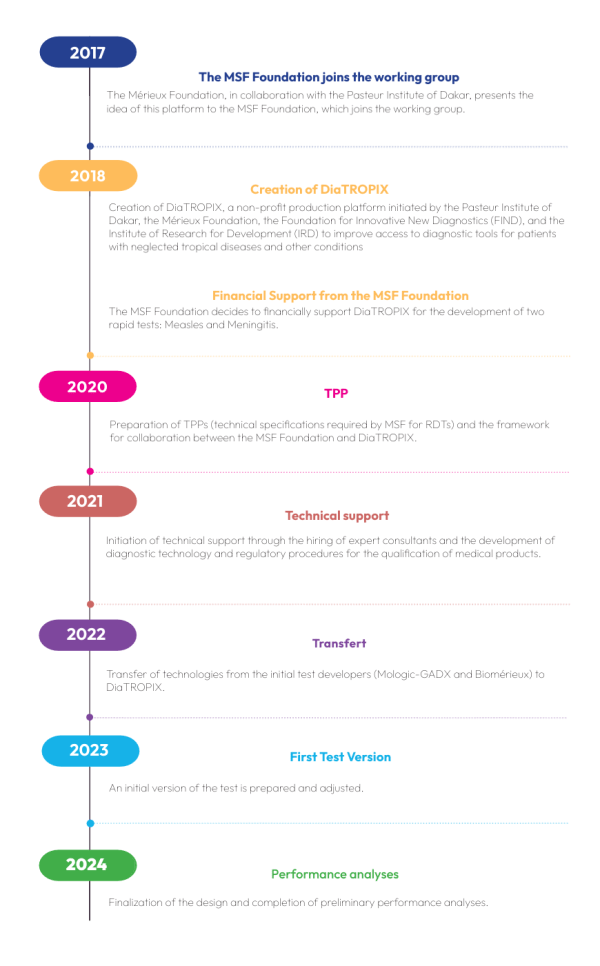
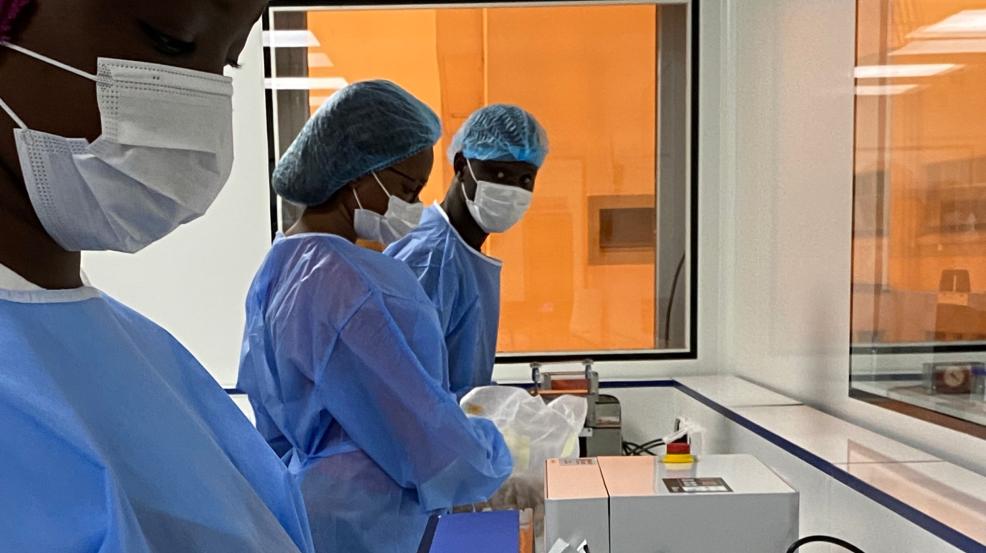
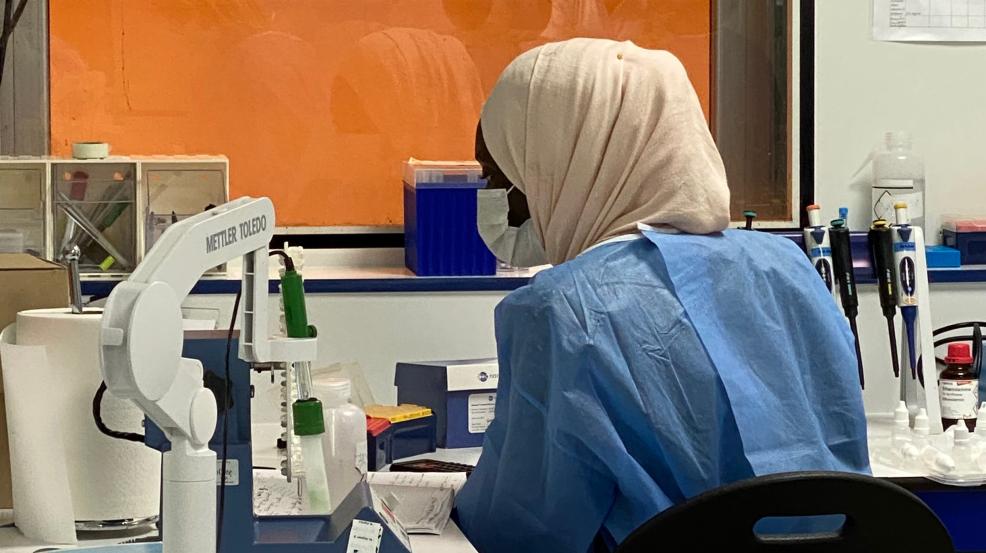
DiaTROPIX is a platform for the development and production of rapid diagnostic tests (RDTs) within the Pasteur Institute of Dakar. This non-profit initiative aims to produce new rapid diagnostic tests for countries where access to laboratory diagnostics is limited or non-existent.
The MSF Foundation finances and supports DiaTROPIX in developing two new RDTs for measles and meningitis. These two diseases, with high epidemic potential, represent a significant public health issue in the countries where MSF runs medical programs, particularly in Sub-Saharan Africa.
In 2025
Measles RDT – The test design has been finalized, and the production of validation and verification batches is underway. The next step will be conducting analytical and clinical studies to evaluate the test's performance and stability. This is in preparation for regulatory validation and future commercialization at an affordable cost for healthcare facilities in low-resource settings.
Meningitis RDT – Adjustment of the transferred prototype is in progress.
Status of the project
News
In detail
The MSF Foundation and the DiaTROPIX initiative
DiaTROPIX is a non-profit production platform created in 2018 at the initiative of the Pasteur Institute of Dakar, the Mérieux Foundation, the Foundation for Innovative New Diagnostics (FIND), and the Institute of Research for Development (IRD) to improve access to diagnostic tools for patients with neglected tropical diseases and other conditions. DiaTROPIX aims to develop and produce prototypes that do not fit into a for-profit business model but have a demonstrated impact on improving patient care in low-income countries.
In 2017, the Mérieux Foundation, in collaboration with the Pasteur Institute of Dakar, presented the idea of this platform to the MSF Foundation, which joined the working group to explore the subject further, assess its feasibility, and list MSF's operational needs.
Being located in West Africa and supported by the Pasteur Institute of Dakar allows the development of tests close to the patients affected by these deases.
The impact of rapid diagnostic tests on epidemic response
These two rapid tests will:
- Provide reliable and quality diagnostics to vulnerable populations and regions where these epidemics are endemic and previously lacked access. They are adapted to precarious contexts in low-resource countries and can be used by non-expert health personnel.
- Enable earlier identification of cases and the pathogen. Epidemic response is a race against time; this time-saving and precision will allow for targeted, effective, and faster intervention.
- Indicate to health personnel when it is necessary to send samples to the laboratory for confirmation.
Providing access to quality diagnosis in precarious contexts
RDTs have proven their effectiveness, yet the RDTs available on the market or in production do not cover all diseases with epidemic potential. Why?
To produce an RDT, significant purchase volumes must be ensured, requiring a "market" that allows laboratories to make a profit. Developing an RDT is long and costly (requiring prototyping, clinical trials, evaluations, etc.). For measles and meningitis, this market does not exist. If the MSF Foundation and initiatives like DiaTROPIX do not create it by pre-ordering and funding this research, these tests would never be available.
By defining the characteristics of the tests we need, then using and evaluating these products in the field, MSF will demonstrate the urgent need for their existence and continued use in the contexts where we work. By engaging in the pharmaceutical production ecosystem, the MSF Foundation can stimulate the acceleration of the regulation and commercialization of these tests and encourage donors to fund their purchase for use in low-income countries.
The role of the MSF Foundation
The MSF Foundation has chosen to finance and support DiaTROPIX in developing two new rapid diagnostic tests (RDTs) for measles and meningitis. In addition to funding, it also collaborates with MSF medical teams to provide clinical evaluations of these tests in precarious intervention settings. Finally, close collaboration with the Pasteur Institute of Dakar is ongoing for the qualification of these tests with the WHO to make them available more quickly.
Today, a prototype has already been manufactured, tested, and evaluated by MSF teams and the Pasteur Institute of Dakar. The teams are actively preparing for large-scale production and deployment.
The Main stages of the program