VACCINE MICROPATCHES
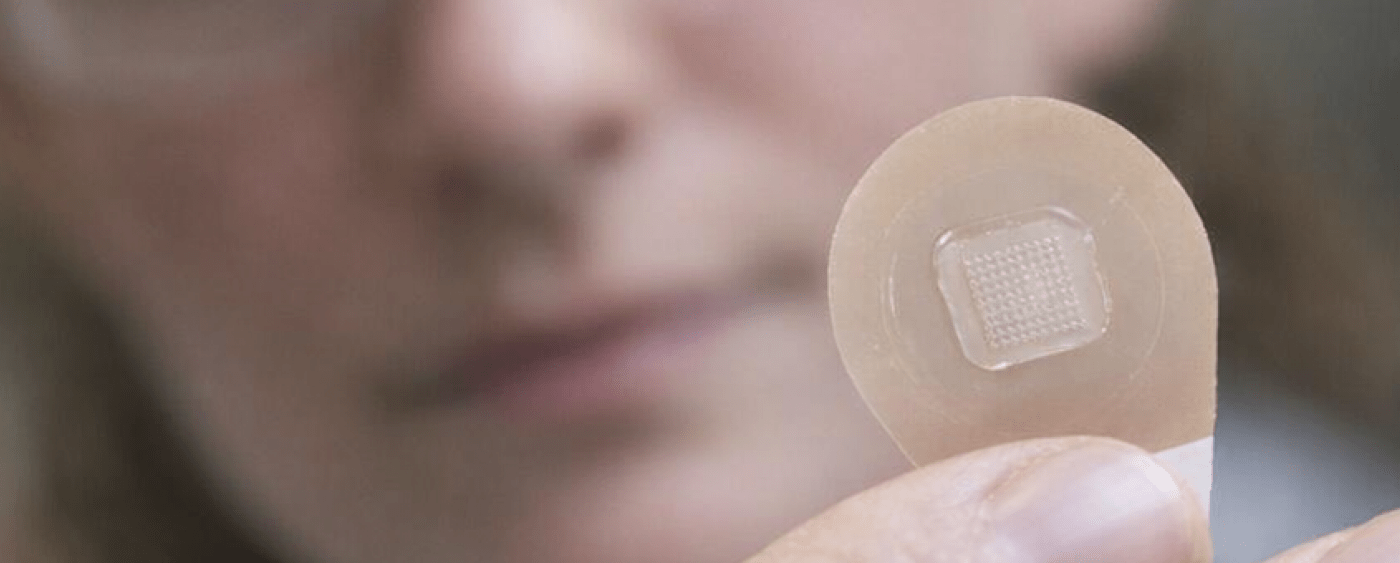
An innovation
Measles remains one of the leading causes of preventable child mortality in low- and middle-income countries, with nearly 100,000 deaths per year.
Despite the availability of an effective vaccine, overall vaccination coverage remains too low to significantly reduce case numbers and prevent outbreaks. Although the organisation regularly organises vaccination campaigns, these campaigns remain complex to implement, particularly due to the need for qualified personnel, the use of needles and syringes, waste management, and cold chain constraints.
To address these obstacles, MSF is exploring a promising innovation: vaccine micropatches, or Microarray Patches (MAPs), which allow vaccines to be administered through the skin without needles or syringes.
MSF's commitment
Since 2018, MSF has been closely following the development of this technology, which could transform the approach to vaccination in unstable contexts. Three candidates for a combined measles-rubella vaccine are currently at different stages of clinical trials.
These micropatches would offer several advantages:
- Painless for the patient -> increased acceptability
- Simplified use → potentially even by non-medical personnel
- No needles or syringes → lower risk of contamination and less waste
- Improved thermal stability → use outside the cold chain to overcome many logistical problems that hinder vaccination efforts
With approval for market release expected by 2030, MSF and the MSF Foundation want to ensure that these devices are operational as soon as they are launched, particularly in contexts of high need: epidemics, remote areas, children under 9 months of age, and displaced populations.
The role of the MSF Foundation
With its expertise in medical innovation and its proximity to the association's areas of intervention, the MSF Foundation plays a central role in representing MSF in the development of micropatches and ensuring that they are suitable for humanitarian contexts.
In its influential position, it maintains an ongoing dialogue with key players in the sector – manufacturers, researchers, health institutions – to ensure that MSF's operational needs are taken into account in the next stages: product design, definition of implementation conditions, and recommendations for use. It also ensures that populations often excluded from initial clinical trials are included in future research phases.
As part of a WHO call for proposals, the MSF Foundation is supporting Epicentre in conducting a pre-implementation study on the use of micropatches in humanitarian settings. The study will take place in the Democratic Republic of the Congo over an 18-month period. Its aim is to assess what a micropatch-based vaccination campaign could look like and the conditions needed for its rollout, with the goal of turning this innovation into a practical solution and strengthening vaccine equity in the most vulnerable contexts.

Markel Redondo
